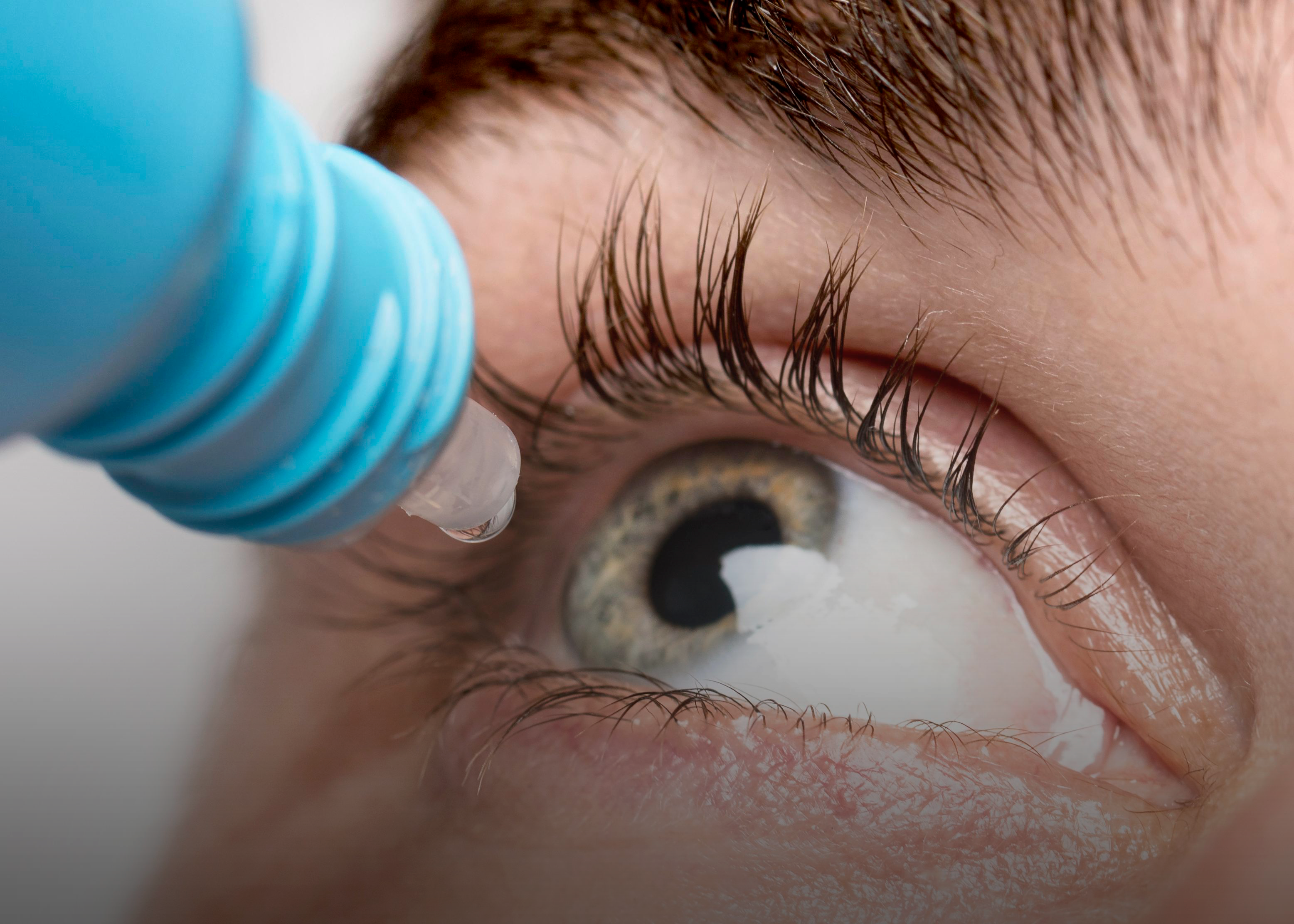
An outbreak of infections caused by drug-resistant bacteria that have led to significant health concerns across the United States came from multiple eye drop products:
- Dr. Berne’s MSM Drops 5% Solution
- LightEyez MSM Eye Drops Eye Repair
The FDA has identified bacterial and fungal contamination in these products:
- LightEyez variant found to be contaminated with drug-resistant bacteria strains such as Pseudomonas aeruginosa, Mycobacterium, Mycolicibacterium, and Methylorubrum
- Dr. Berne’s product was found to be contaminated with Exophiala fungi.
Notably, both products contain methylsulfonylmethane (MSM) as their active ingredient, a substance not commonly found in legally marketed eye drugs within the U.S.
For more information read:
Contaminated Products
Dr. Berne’s MSM Drops 5% Solution and LightEyez MSM Eye Drops Eye Repair found to have bacterial and fungal contamination.
Identified Bacteria Strains
Pseudomonas aeruginosa, Mycobacterium, Mycolicibacterium, Methylorubrum, and Exophiala fungi detected in the mentioned eyedrop products.
Active Ingredient
Both products contain methylsulfonylmethane (MSM). These products are unapproved drugs and illegally marketed in the U.S. There are no legally marketed ophthalmic drugs that contain MSM as an active ingredient.
Another Recall By The FDA
Another recall was initiated following an investigation by the FDA that revealed unsanitary conditions in the manufacturing process of Kilitch Healthcare India Limited eye drops. The potential risks associated with using these products include the increased likelihood of eye infections or other related harms.
Products Affected
As more research has been conducted and test results have been concluded, the following products have been found to contain the pseudomonas aeruginosa bacteria that is causing the associated health and vision issues:
- Clear Eyes Once Daily, Eye Allergy Itch Relief
- Purely Soothing 15% MSM Drops
- Brimonidine Tartrate Ophthalmic Solution
- Dr. Berne’s MSM Drops 5% Solution
- LightEyez MSM Eye Drops-Eye Repair
- CVS Health Lubricant Eye Drops 15 ml (single pack)
- CVS Health Lubricant Eye Drops 15 ml (twin pack)
- CVS Health Lubricant Gel Drops 15 ml (single pack)
- CVS Health Lubricant Gel Drops 15 ml (twin pack)
- CVS Health Multi-Action Relief Drops 15 ml
- CVS Health Lubricating Gel drops 10 ml
- CVS Health Lubricant Eye Drops 10 ml (single pack)
- CVS Health Lubricant Eye Drops 10 ml (twin pack)
- CVS Health Mild Moderate Lubricating Eye Drops 15 ml (single pack)
- Rugby Lubricating Tears Eye Drops 15 ml
- Rugby Polyvinyl Alcohol 1.4% Lubricating Eye Drops 15 ml
- Leader Dry Eye Relief 15 ml
- Leader Lubricant Eye Drops 15 ml (single pack)
- Leader Dry Eye Relief 10 ml
- Leader Eye Irritation Relief 15 ml
- Rite Aid Lubricant Eye Drops 15 ml (twin pack)
- Rite Aid Lubricant Eye Drops 10 ml (twin pack)
- Rite Aid Gentle Lubricant Gel Eye Drops 15 ml
- Rite Aid Lubricant Gel Drops 15 ml
- Rite Aid Lubricating Gel Drops 10 ml
- Rite Aid Multi-Action Relief Drops 15 ml
- Target Up&Up High Performance Lubricant Eye Drops 15 ml
- Target Up&Up High Performance Lubricant Eye Drops 15 ml (single pack)
- Target Up&Up Dry Eye Relief 15 ml
- Velocity Pharma LLC Lubricant Eye Drop 10 ml (triple pack)
- Walmart Equate Hydration PF Lubricant Eye Drop 10 ml
- Leader Lubricant Eye Drops 15 ml (twin pack)
The following companies that voluntarily recalled their products and have since been cleared/vindicated of being the source or being connected to the contamination issues of any kind include:
- EzriCare Artificial Tears Lubricant Eye Drops
- Delsam Pharma Artificial Tears
- Delsam Pharma Artificial Eye Ointment